Skaffold
-
What do the most widely held stocks in Australia all have in common?
Roger Montgomery
June 29, 2012
EXCLUSIVE CONTENT
subscribe for free
or sign in to access the articleby Roger Montgomery Posted in Financial Services, Skaffold, Value.able.
- 9 Comments
- save this article
- POSTED IN Financial Services, Skaffold, Value.able
-
Your Exclusive Guide to Understanding Biotechs.
Roger Montgomery
May 31, 2012
Praveen has really put in some effort to bring this to you. Enjoy!
ANATOMY OF A BIOTECH by Praveen Jayarajan 1st May 2012
Investing in biotechnology companies is an inherently risky endeavour for many reasons, with many pundits of the view that the entire sector is highly speculative. But as with any such investment, the potential returns can be huge. However, the average investor, who may not be familiar with the terminology used in company reports, presentations, analyst coverage, and the media, may not have a great deal of understanding about the industry and what these companies actually do. In this article I am going to try and decode and de-jargon the industry and hopefully give you a better understanding of how to assess these companies.
Big Pharma vs Biotech
To begin with, we need to appreciate the traditional differences between a pharmaceutical and a biotechnology company. Traditionally, pharmaceutical companies, also now commonly referred to as “Big Pharma”, have been involved in small molecule therapies. What this means is that the drugs they produce are made from molecules that are relatively simple and small in size. These molecules are all chemically manufactured or synthesized by combining different compounds in a laboratory. They are also available in the form of an oral tablet or capsule, and can be easily absorbed into the bloodstream through the intestine. Once in the bloodstream they are able to penetrate different cells in the body due to their small size. An example is Lipitor, used to treat high cholesterol, made by Pfizer.
In contrast, biotechnology companies, also referred to as “Biotech”, have been involved in large molecule therapies called biologics that are based on molecules that are more complex and large in size. These molecules are manufactured using genetically modified living cells of microorganisms such as viruses and bacteria, as well as from human and animal sources. Biologics are usually administered via an injection or infusion, as they are too big to be absorbed when given orally. Once in the bloodstream they act in different ways to traditional drugs, including binding to receptors on the surface of cells rather than penetrating the cells. An example is Fluvax, which is the influenza vaccine, made by CSL.
The line between these two definitions has become increasingly blurred. In fact, these traditional definitions for Big Pharma and Biotech do not have as much relevance today. Biotech’s are now seen as smaller research and development companies, with Big Pharma taking the role of the larger company with the expertise and funding to progress a drug or biologic (in this article I will may also use the word “product” or “medication” interchangeably when referring to drugs and biologics) through the regulatory process and then take it into production and marketing. Examples of Big Pharma include Pfizer, Roche, GlaxoSmithKline, Novartis, Sanofi, and AstraZeneca. Note that there are also companies that can be loosely considered “Small Pharma” and “Big Biotech”.
The Patent Cliff
What is a patent? A government license that gives the holder exclusive rights to a process, design or new invention for a designated period of time. Applications for patents are usually handled by a government agency (taken from Investopedia).
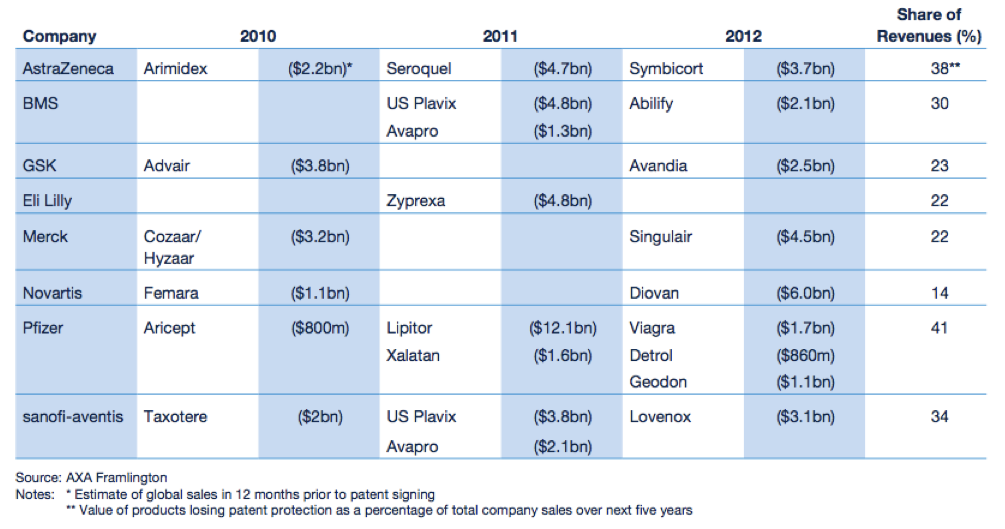
The “patent cliff” refers to what happens to revenues when an original product’s patent expires. As this happens, in the case of Big Pharma, they face competition from similar drugs made by other companies at a fraction of the price. These drugs are called generics. Generic drug manufacturers need to prove that their version of the drug is bioequivalent to the original. That is, it must have the same active ingredient and have the same properties as the original, but it does not have to go through extensive clinical trials like the original. According to the IMAP Pharma and Biotech Industry Global Report (2011), original drugs can face a price erosion of 70% within months of going off patent. Furthermore, revenues of drugs going off patent between 2010 and 2014 will be ~ US$89.5 billion. A number of “blockbuster” drugs (drugs that have revenues greater than US$1 billion) are due to go off patent in the coming years. The world’s biggest selling drug Lipitor, went off patent towards the end of 2011.
With regards to biologics, the patent cliff issue still applies, off patent biologics are called biosimilars or follow-on biologics. The difference is that because biologics are complex molecules, it is much harder and more expensive to make a bioequivalent version, and the regulatory requirements are far more rigorous. The worldwide biosimilars market is still in its infancy, with only a handful on the market. Note that there are also “me-too” products, these have similar active ingredients and mechanism of action to the original product, and almost identical clinical outcome, but unlike generics/biosimilars, may have other benefits. This could be increased efficacy, a different profile of adverse affects, or lower costs.
Figure 1: Expected fall in revenues for Big Pharma
Source: Pharma 2020 Report PWC
The R&D Pipeline
The research and development (R&D) pipeline refers to the group of drugs or biologics that a particular company is aiming to take into production and market. Generally, the more products it is developing and the later they are in the development phase, the more likely it is that one of their products will eventually be produced and marketed. In the 1990s, the discovery of a raft of blockbuster drugs resulted in huge revenues for the companies now recognised as Big Pharma. However, in recent times the efficiency or rate of return on their R&D efforts have been declining, with less products in late-stage development, higher average costs to develop and bring a drug to market, and a dearth of blockbusters to replace the ones going off patent. As a result of this Big Pharma are looking for innovation in the form of new biologics from their brothers in Biotech.
Big Pharma and Biotech collaboration
There is now increasing partnership and merger & acquisition (M&A) activity between Big Pharma and Biotech. Both sides have something to gain in this collaboration. Big Pharma are able to grow their pipeline, and with Biotech able to get the necessary funding to continue their R&D. With partnerships or “in-licensing”, Big Pharma will usually enter into an agreement with a Biotech whereby the Biotech will receive an upfront payment, and then further “milestone” payments as they advance the development of the product. Once the product is on sale, the Biotech will also receive royalty payments. When Big Pharma partner with a Biotech that has a product in the very early stages of development, the Biotech is likely to receive a lower share of royalties, whereas if the partnership occurs at an advanced stage, the Biotech is more likely to receive a larger share of royalties. With M&A activity, Biotech’s are being bought out at significant premiums, with the best companies fielding several bids from cashed up and hungry Big Pharma’s.
Regulatory affairs
Across the world, in order for a Big Pharma or Biotech company to be able to market or sell it’s product, the product needs to have been approved by the relevant government regulatory agency in each region that it plans to sell the product. In the US, this is the Food and Drug Administration (FDA), and in Europe, Japan, and Australia, this is the European Medicines Agency (EMA), the Ministry of Health Labour & Welfare Japan, and the Therapeutic Goods Administration (TGA) respectively. The International Conference on Harmonisation (ICH) was a project that brought together the regulatory agencies in the US, Europe, and Japan with the view to harmonising the regulatory requirements across these regions and the world. The ICH developed guidelines for regulation that have been adopted by certain countries, or are very similar to existing guidelines in others. As such, once a product has been approved in one country, meeting regulatory requirements for approval in others can be relatively straightforward.
The US FDA approval process
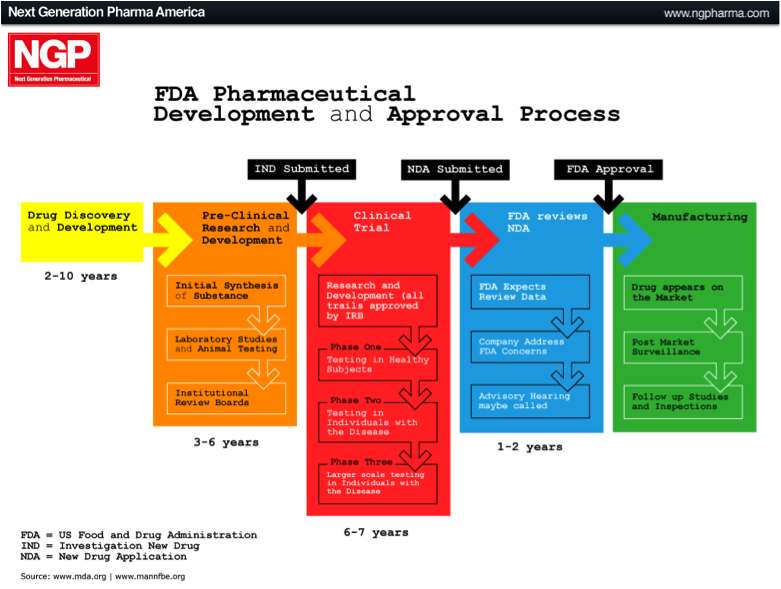
The largest market in the world for drugs and biologics is the US. It is the Holy Grail if you like, which is why many international companies tailor their product development towards meeting the FDA requirements and getting FDA approval. The approval process can be broken down into different phases, as outlined below.
Pre-Clinical Phase: This phase begins after the discovery of new molecules, and is where new molecules are investigated and tested in the laboratory (in-vitro), on animals (in-vivo), or via computer simulation (in-silico). Once this phase is completed the company files an Investigational New Drug Application (IND).
Phase 1: Once the FDA has approved the IND, Phase 1 human testing begins. In this phase the product is tested on a small group of healthy individuals to assess it’s safety. This means testing to make sure it is not toxic or carcinogenic, and to determine appropriate doses. It is also assessed to see how it is broken down or metabolised by the body.
Phase 2: Here the product has been established as safe in humans and is now tested on a small group of individuals that are affected by the disease being targeted. This is done to see if the therapy actually works, as well as any short-term effects.
Phase 3: In this phase, often referred to as the late-stage in development, the product is tested on a much larger population. The trial is generally multicentre, double-blinded, randomised and placebo-controlled (i.e. a randomised controlled trial, RCT). This means that testing is conducted at different centres and neither the subject nor the physician know whether the actual product or a placebo is being given, and the allocation is completely randomised.
At the completion of Phase 3, if the product has been proven to be safe and effective, the company will apply for a New Drug Application (NDA) or Biological License Application (BLA). If this is accepted, the final product can be marketed.
Complete Response Letter (CRL): The FDA issues a CRL in response to an NDA/BLA. In essence it is a “please explain”, that requires the applicant to clarify issues that may relate to the product, including safety, efficacy, or manufacturing related issues. In some cases the issues are minor, in other cases they could results in rejection of the application.
Figure 2: US FDA approval process
Source: Next Generation Pharmaceutical
Special Protocol Assessment (SPA): This is granted to products in uncompleted Phase 3 trials that have design, clinical endpoints/outcomes, and statistical analyses that are acceptable for approval by the FDA. The SPA is an indication that if the trial goes to plan, the final product will be approved.
Prescription Drug User Fee Act Date (PDUFA Date): PDUFA is a law that allows the FDA to collect fees from product manufacturers in order to fund the approval process. The PDUFA Date refers to the deadline by which the FDA must either approve or reject an NDA/BLA. The FDA has 10 months to review new applications.
Fast Track / Accelerated Approval / Priority Review: The FDA grants a quicker approval for important products. Fast Track is for those that treat serious diseases (e.g. AIDS, Cancer) AND fill an unmet medical need (i.e. provide a treatment where nothing else exists, or that is superior to existing treatments). Fast Track designation enables eligibility for Accelerated Approval. Accelerated Approval involves using a surrogate endpoint, e.g. a laboratory measurement or physical sign used in a clinical trial as an indirect measure of clinical outcome (survival or symptom improvement). Priority Review is used when products offer again treatment where nothing else exists, or more superior treatment, but it can be for serious or non-serious diseases, and approval can be obtained within 6 months.
Orphan Product Status: Orphan product status or designation is granted to products that treat rare diseases or conditions. The orphan designation offers a number of advantages, including product tax credits, marketing incentives, and a period of exclusivity.
Phase 4: In this phase the product is already on the market and ongoing monitoring is undertaken to observe for any new short-term or long-term effects. In some cases new data has resulted in products being taken off the market, most notably in the case of the drug Vioxx marketed by Merck & Co. Vioxx was a widely used anti-inflammatory drug used to treat acute and chronic pain related to arthritis. It was found to increase the risk of heart disease and stroke. Prior to withdrawal it had revenues of ~US$2.5 billion and was used in over 80 million people.
Summary: Drug and biologic development is a lengthy process, certainly an 8 to 10 year period from beginning the search for a potential product to final FDA approval is quite common. This requires significant funding. In assessing a Biotech one needs to be acutely aware of the amount of cash they have in reserve as well as the cash burn rate (how much cash they are spending each year). According to the latest AUSBiotech Biotechnology Industry position survey (April 2012), 34% of those surveyed have less than 12 months cash on hand at current burn rates, and 46% intend to raise capital in the coming year. However, raising funds from equity/debt capital markets or even venture capital funds may be difficult for Biotech companies in the current economic climate. Companies that may fare better are those that are already partnered with Big Pharma, or at the least are able to attract a premium valuation for acquisition.
THE PROVIDER-PAYER RELATIONSHIP, PRICING, & REIMBURSEMENT
Once a company (the provider) gets it product approved by the relevant government regulatory agency, it needs to also get it listed on a formulary. A formulary is a list of medications. Private health insurers (the payer) have a formulary. This is a list of the medications that they will pay for, usually with a part contribution or “co-payment” from the person who has been prescribed the medication. More importantly, the provider needs to get its product on a government formulary (another payer). In the US this is the Medicare Part D and Medicaid formulary. Here in Australia it is the Pharmaceutical Benefits Scheme (PBS). Private health insurers generally only cover medications already listed on a government formulary. Payers (private and public) can take up to 9 to 12 months to add new products to their formulary. In Australia, once a product has TGA approval the Pharmaceutical Benefits Advisory Committee (PBAC), an independent expert government body, makes a recommendation (to the Health Minister) as to whether it should be listed on the PBS. Once that recommendation has been accepted the Pharmaceutical Benefits Pricing Authority (PBPA) determines what price the government should pay for the medication. This is then negotiated with the provider. As an example of how the reimbursement process then works, we can look at how medications are dispensed from a pharmacy. A wholesaler first purchases the product or medication from the provider and then sells it to the pharmacy. The pharmacy receives the patient’s co-payment once the medication is dispensed, and then they receive reimbursement for the remainder of their costs from the government. This reimbursement includes the provider’s price for the medication + wholesaler’s mark-up + pharmacy mark-up + dispensing/other pharmacy fees.
THE IMPACT OF HEALTH POLICY
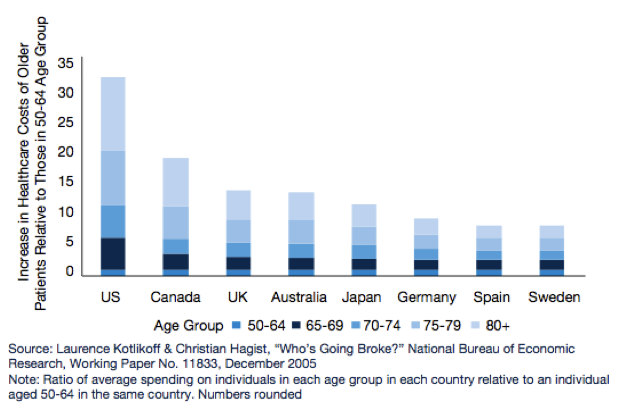
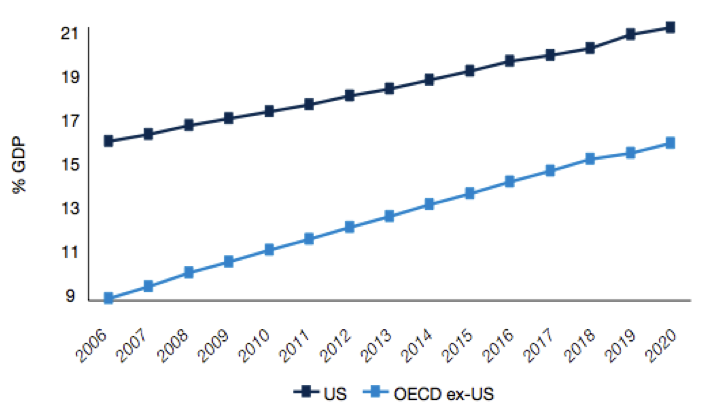
Based on a number of surveys done across the world, what we know about the developed world’s population is that it is ageing. What this means for government’s around the world is that healthcare and pharmaceutical related expenditures are going to rise significantly over the coming years. This will impact government health policy and in turn it will impact pharmaceutical and biotechnology companies.
Figure 3: Increased healthcare costs for older people
Source: Pharma 2020 Report PWC
Figure 4: Rising health expenditure as a % of GDP
Source: Pharma 2020 Report PWC
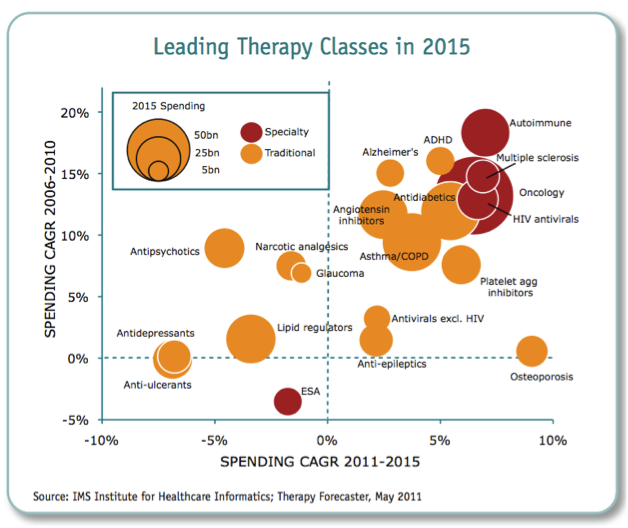
What we could therefore see is tighter regulation of products, shorter periods of exclusivity for original product manufacturers, lower drug prices or reimbursement, and more emphasis on lower cost generics or biosimilars. According to the IMS Institute for Health Informatics (2011), the key drivers for growth in the future will be increased spending from emerging markets (led by China), with a focus on generics or biosimilars, and specialty medicines (biologics).
Figure 5: Leading therapeutic classes in 2015
Source: IMS Institute of Health Informatics 2011
GRADE Classification of BioTechs
In reviewing Biotech companies I like to classify them according to 4 Grade’s, albeit arbitrarily. This is a way of trying to differentiate companies according to the strength of their product pipeline. There’s no science behind it, and it is just a guide or a starting point for further investigation, which you may or may not find useful.
Grade 1: At least 3 products on the market.
Grade 2: 1 or 2 products on the market, with at least 1 other in Phase 2 or 3 trials.
Grade 3: 1 product on the market, or no products on the market but at least 1 in Phase 3.
Grade 4: No products on the market, multiple in Pre-Clinical Phase, Phase 1, or Phase 2.
Across the Grade’s, Grade 1 companies could be considered relatively low risk as they already generating revenues and cash flow. On the other hand, these companies may have already experienced high levels of capital growth off the back of their existing products and may not have as much room to climb further, depending a lot of course on their expected future cash flows and pipeline. They may also be faced with the issue of expiring patents and increased competition from new products. I’d expect the most gains to be achieved from investing in a successful Grade 4 company that is held for the long-term. However, with the exceptionally long time it takes for product development, at the Grade 4 stage it is very hard to assess the likelihood for long-term success. Furthermore, success in Phase 1 or 2 trials is not necessarily a reliable indicator of Phase 3 success or likelihood for regulatory approval. The sweet spot for me is going to be Grade 2 and 3. In order to maximise the potential returns on a Biotech investment, I want to capture those that are closer to their “inflection point”, which may be in these mid or lower ranked Grade’s.
The problem here is that many of these companies are yet to generate any revenues, so a reliable valuation is difficult to make. However, using Skaffold (http:///www.skaffold.com), we can at the very least start by filtering out the ASX-listed Biotech companies, and then find those that are forecast to have a rise in their future intrinsic value. In the table below I have extracted and listed all Healthcare sector companies that that are also part of the Biotechnology/Major Drugs industry groups. The companies listed below all have analyst coverage. I have excluded 2 companies that did not really fit into the defined Grade classification, Mayne Pharma (diversified pharmaceutical services), and Sigma Pharma (wholesale and retail pharmaceutical distribution/sales).
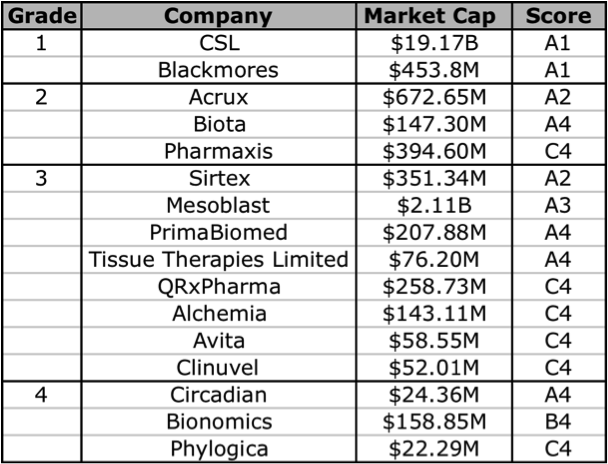
Figure 6: Skaffold biotechnology company current Quality Scores
Source: Skaffold 26 April 2012
Based on the Skaffold Scores, there are 5 companies that could be considered investment grade. They are the A1, A2, and A3 companies: CSL, Blackmores, Acrux, Sirtex, and Mesoblast. Although Mesoblast does not have any intrinsic value, as it does not have any current product revenues. Mesoblast is an interesting one, although it has a lot of cash, this is largely the result of a partnership with Cephalon (now Teva). There are a handful of other companies that have analyst forecasts for near-term product revenue and thus have a future intrinsic value: Biota, Tissue Therapies Limited, Bionomics, Alchemia, Pharmaxis, and QRxPharma. In the following sections I will give a brief overview of 2 companies that currently have products on the market, Acrux and Sirtex, note that the former makes products that enable drug-delivery, and the other is technically a medical device company.
ACRUX (ASX:ACR)
Acrux has a number of products in its pipeline, all of which are based on transdermal delivery. That is, the medications are absorbed into the bloodstream through the skin via sprays, gels, or solutions. The medications themselves have all already been used safely when administered in other ways, for example, via tablets or injections.
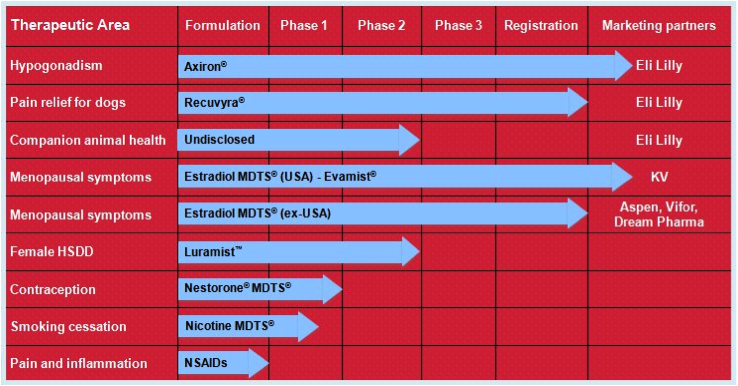
Figure 7: Acrux R&D pipeline
Source: Acrux website
Their lead product is Axiron. Axiron is a testosterone hormone solution used to treat low testosterone hormone levels in men (hypogonadism). It is applied in the armpits using an applicator, much like a roll-on deodorant. It was given FDA approval in 2010 and is on the market in the USA. Acrux signed a global licensing deal with Big Pharma Eli Lily in 2010 for the marketing of Axiron. As part of this deal, Acrux is entitled to up to $US335 million in milestone payments as well as worldwide royalties. The partnership with Eli Lily appears like an ideal fit, with Eli Lily already involved in the men’s health market with their erectile dysfunction product Cialis (a competitor to Pfizer’s Viagra). Axiron is actually the second product that Acrux has progressed to FDA approval, the previous being Evamist in 2007, an oestrogen hormone spray used in menopausal women that is also on the market in the USA. Acrux has also ventured into animal health products with Recuvrya, a pain relief solution for dogs that was only recently approved by the EMA (in 2011).
But let’s get back to Axiron. Low testosterone can result in symptoms such as erectile dysfunction, as well as decreased libido, energy, and mood. It is found in people who have a genetic or acquired disorder of the testes (where testosterone is produced) or of the pituitary gland in the brain (where testosterone levels in the bloodstream are regulated or controlled). Low testosterone levels can also be associated with many chronic illnesses, such as obesity, heart disease, and even depression. These illnesses may cause symptoms similar to that of testosterone deficiency, but this does not necessarily mean that testosterone replacement is required. Often focussing on treatment of the underlying illness is what is required instead. Testosterone levels also naturally decline with age, however this is gradual and usually does not get to the point where treatment is required either.
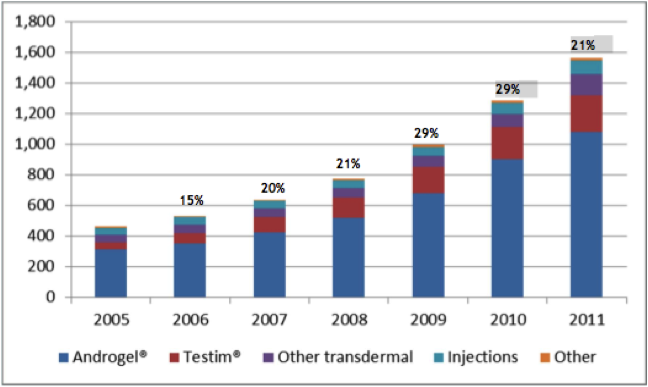
Now there are various testosterone products on the market, with many using different delivery methods, however the recent trend is favouring the newer transdermal routes. When testosterone is given in oral form, it is very difficult to achieve effective and stable concentrations in the bloodstream, this is because after it is absorbed through the intestine it is broken down or metabolised by the liver rapidly. In injectable form (a deep intramuscular injection, e.g. in the buttock) it has to be given every 1 to 3 weeks, this can be associated with severe fluctuations in testosterone concentrations in the bloodstream. This can lead to fluctuations in symptoms such as libido, energy, and mood. In the transdermal market, testosterone patches (think along the lines of nicotine patches) have had anectodally large incidences of severe skin rashes that often result in treatment cessation. This is where the gel/solution based products such as Axiron, Androgel, Testim, and Fortesta are increasingly popular. The overall male testosterone market, in particular for transdermal therapy, has been steadily increasing over the last 6 years as illustrated in the graph below.
Figure 8: Male testosterone market, US 2005-2011 (millions)
Source: Acrux February 2012 Presentation / IMS Data
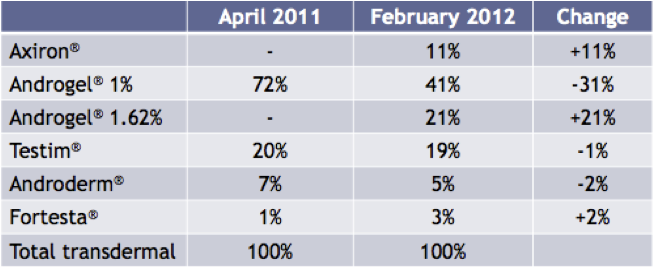
More importantly though is how Axiron has fared since coming onto the market in April 2011. As of February 2012, Axiron has achieved an 11% share of the transdermal market. Axiron’s main rival product in this market is another Big Pharma product Androgel, from Abbott Laboratories. In this same period, Androgel’s total share of the transdermal market has fallen by 10%, as illustrated in the chart below.
Figure 9: Share of Total Prescriptions of transdermal products in the USA
Source: Acrux February 2012 Presentation / IMS Data
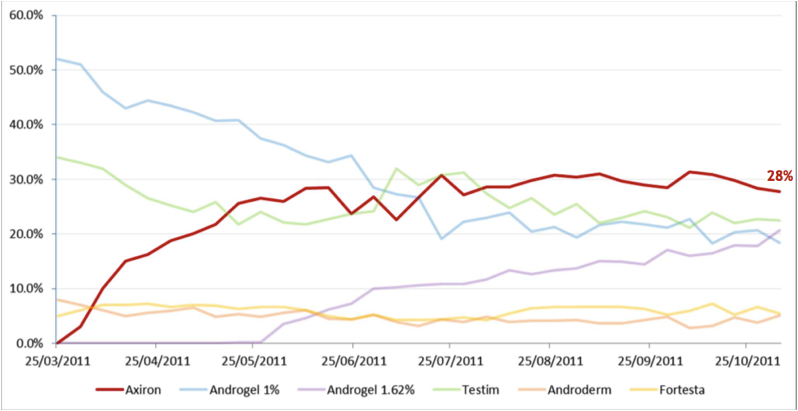
The following graph shows the different transdermal products in terms of their share of total new prescriptions for patients being initiated on treatment for the first time by specialists, or being switched from other products. The most obvious thing to note is how in a short space of time Axiron has become the most widely prescribed product, and that the percentage share of total new prescriptions for Androgel has been declining significantly.
Figure 10: Share of transdermal New to Brand Prescriptions by Specialists
Source: Acrux Novembery 2011 Presentation / IMS Data
It appears that the uptake of Axiron has been excellent, and has the potential to eat away an even larger chunk of the total transdermal market. A key advantage of Axiron over Androgel is that is applied to an area that is less likely to come into contact with other people. Androgel on the other hand is applied over the upper arms and shoulders, so has a much greater chance of being indirectly transferred to other people. Another advantage of Axiron is that you do not need to physically touch the solution with your hands, whereas with Androgel you do have to rub the gel directly onto the skin with your hands.
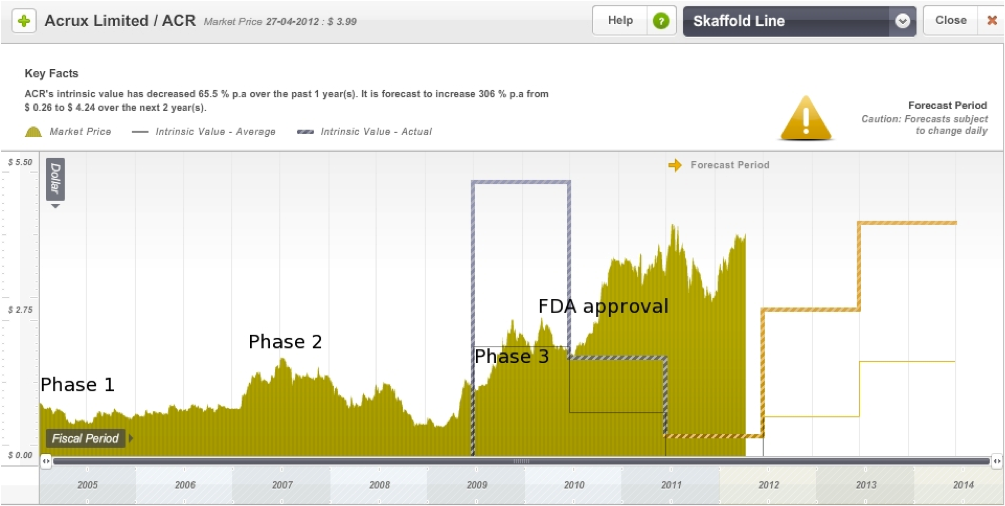
On the Skaffold line graph below I have roughly marked out the Phase 1, 2, and 3 announcement dates as well as the date of FDA approval for Axiron. Acrux is currently trading at a significant premium to intrinsic value, however the intrinsic value is expected to rise significantly in the next few years.
Figure 11: Acrux Skaffold Estimated Intrinsic Valuation line
Source: Skaffold 27 April 2012
SIRTEX (ASX:SRX)
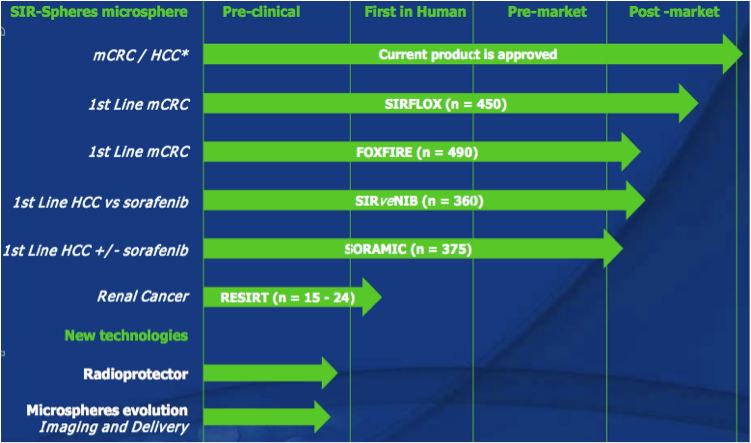
Sirtex has one product on the market, SIR-Spheres microspheres (let’s called it SIR-Spheres from now), which is a treatment option available for advanced hepatocellular carcinoma (“HCC”, liver cancer/tumour(s) that cannot be surgically removed), as well as for metastatic colorectal carcinoma (“mCRC”, bowel cancer that has spread to the liver, where the liver cancer/tumour(s) cannot be surgically removed). Sirtex also has a number of products or technologies in pre-clinical phase, although not a lot of information is available about them as they are at such an early phase.
Figure 12: Sirtex R&D pipeline
Source: Sirtex Company Presentation October 2011
SIR-Spheres is a radioactive treatment. Sirtex have developed micro-particles or beads (made from a substance called Resin) that act as transport vehicles for an isotope called Yttrium-90. An isotope is a radioactive chemical element. SIR-Spheres can be injected into the hepatic artery (the blood vessel that supplies the liver), once here the micro-particles get lodged into the walls of the small blood vessels that surround the tumour. This is a treatment that enables radiation to be given to a very localised area, without affecting normal liver tissue or other bodily organs. It is often referred to as selective internal radiation therapy (SIRT), or radioembolisation. It is a highly innovative product, although there is another company called Nordion that has something similar called TheraSphere. TheraSphere has micro-particles made from glass, but it also uses the Yttrium-90 isotope.
Radioembolisation is currently not used as first-line therapy. Let’s looks at HCC and mCRC separately to see exactly where it fits. For HCC, the first-line treatment for advanced inoperable cases, apart from liver transplantation, is a procedure called Radiofrequency Ablation (RFA). This is basically when a needle-like probe is passed into the tumour and an electrical current heats the probe resulting in the tumour tissue being heated up and destroyed. Where this is not possible, Transarterial Chemoembolisation (TACE) can be used. TACE is where chemotherapy is administered directly into the hepatic artery. SIR-Spheres radioembolisation could be used as an alternative to TACE. Having said that, with regards to SIR-Spheres, there is not yet enough clinical evidence or consensus with regards to when and whether to use SIR-Spheres over TACE. Systemic or traditional chemotherapy is used in more severe cases of HCC where there are multiple tumours or the other options are contraindicated in the patient. Systemic chemotherapy can also be associated with a lot of side effects. With mCRC involving the liver, where surgery is not an option, RFA is also used. Hepatic Intra-arterial (HIA) chemotherapy (similar to TACE) can be used as an alternate to systemic chemotherapy, although the benefits of this over newer systemic chemotherapy regimes have not yet been clearly demonstrated. Similarly, the benefits of SIR-Spheres over newer systemic chemotherapy regimes have not yet been clearly established either.
When SIR-Spheres was approved by the FDA, the control group in the Phase 3 trials was being given a systemic chemotherapy regime that has now been superseded by a more effective regime. The key downside to this product is therefore the lack of consensus as to when to use it and the lack of enough clinical evidence over existing treatments. SIR-Spheres has been approved for sale in Australia, Europe, and the USA. FDA approval was obtained in 2002, and this was the time of first commercial sale. So what has happened in the last 10 years? Well, they still have the same product, and the fact that it is still their only product is another downside risk, although they are working on number of clinical trials, with 4 major ones in clinical recruitment. These clinical trials are aimed at increasing the indications for use of the product, to see if they can find evidence to support it’s use in the earlier stages of cancer treatment, as first-line therapy or as an add-on to the newer systemic chemotherapy treatments. All that being said, Sirtex has steadily increased its revenues over the last 5 years.
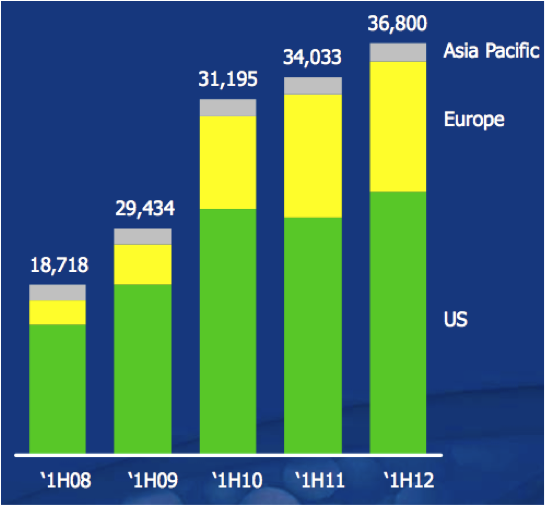
Figure 13: Sales revenues (thousands)
Source: Sirtex February 2012 presentation
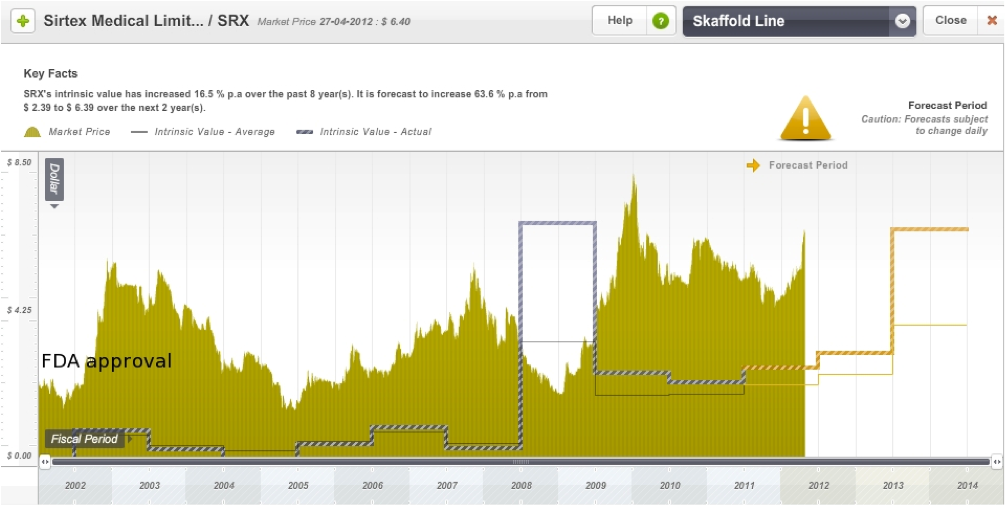
On the Skaffold line below I have roughly marked the date of FDA approval for SIR-Spheres. The share price of the company has been quite volatile over the years, but this is not uncommon in this industry. Like Acrux, it is also trading at a significant premium to its current intrinsic value, however its intrinsic value is also expected to rise in the next few years.
Figure 14: Sirtex Skaffold Estimated Intrinsic Valuation line
Source: Skaffold 26 April 2012
CONCLUSION
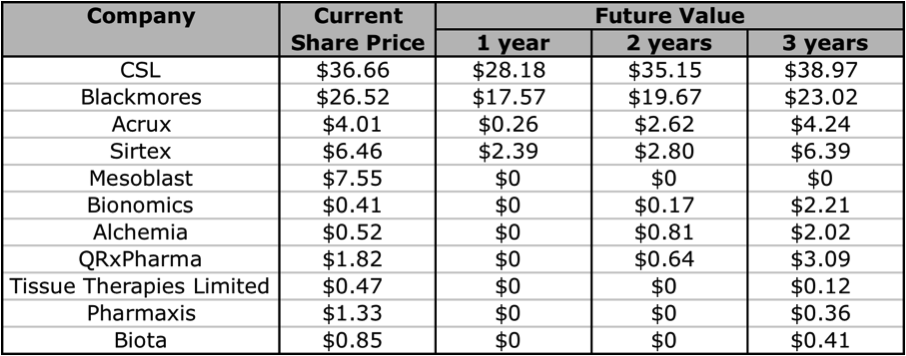
A decision as to whether to invest in either of these companies will require a deeper analysis of their management team, cash flows, debt levels, levels of return on equity, the strength and diversity of their pipeline’s, and industry competition. Unfortunately that is beyond the scope of this article. But if you are looking for an A1 company in this industry, you can’t go past CSL or Blackmores. Both are excellent companies that have had steadily rising intrinsic values for over 10 years. If you want to invest in a company at a much earlier stage, there are a few companies that are worth keeping an eye on. Aside from the 2 I have reviewed in this article, I think Bionomics, Alchemia, and QRxPharma are ones to add to your share portfolio watchlist. Below I have extracted some of the Skaffold data and listed the forecasted future intrinsic values of the companies whose intrinsic values are expected to rise in the next 1 to 3 years. Note: Biota has recently announced that it will be delisting from the ASX, and I have slotted in Mesoblast as an extra.
Figure 15: Currently Estimated Future Values
Source: Skaffold 1 May 2012
Posted for Praveen by Roger Montgomery, Value.able author, Skaffold Chairman and Fund Manager, 31 May 2012.
Do you ‘LIKE’ us?
by Roger Montgomery Posted in Health Care, Intrinsic Value, Skaffold.
- 17 Comments
- save this article
- POSTED IN Health Care, Intrinsic Value, Skaffold
-
Were investors hasty?
Roger Montgomery
May 28, 2012
 It’s true that many Australian businesses are on their own path and even amid the financial tempest raging in Europe there will be companies that succeed.
It’s true that many Australian businesses are on their own path and even amid the financial tempest raging in Europe there will be companies that succeed.Conversely there will many that fail.
I have maintained that beating the market can be made simpler by sticking to high quality issues.
Skaffold’s A1’s, I believe, have the lowest probability of ‘catastrophe’ within a year or two of receiving their score. Of course, you have to realise that business is dynamic and Skaffold’s quality scores will change with new information.
Furthermore, having the lowest probability of catastrophe is not to say there is zero chance of catastrophe. A 100-to-1 nag at Moonee Valley might still win the race.
Nevertheless, I would however rather have a diversified portfolio of A1s and A2s than a portfolio of C5s.
And this brings me to today’s announcement that Hastie Group has voluntarily appointed Administrators, just three days after the company announced accounting irregularities would result in a charge to profits of $20 million.
Hastie Group Limited (HST) has today announced that it has been placed into voluntary administration. Many investors will lose any and all the money they had invested in the company. If you are retired or about to retire, you can least afford such a permanent impairment to your capital.
I get so frustrated when I hear young advisers telling people on air to ‘only use money they can afford to lose’. I don’t know about you, but I cannot afford to lose any!
And that’s were quality comes into its own. Skaffold was designed to mitigate the risk of investing in those companies where the risk of events like the appointment of administrators is highest.
Skaffold’s quality scoring is based on decades of international academic research into forecasting collapses. And for us it just works.
At Montgomery Investment Management the Quality of a company is our first filter. A high Quality Score is the cornerstone to our investment strategy as well as the core of our investment decision-making. The second step is value. We reduce risk even further when we buy high quality companies only when they are at sharp discounts to intrinsic value.
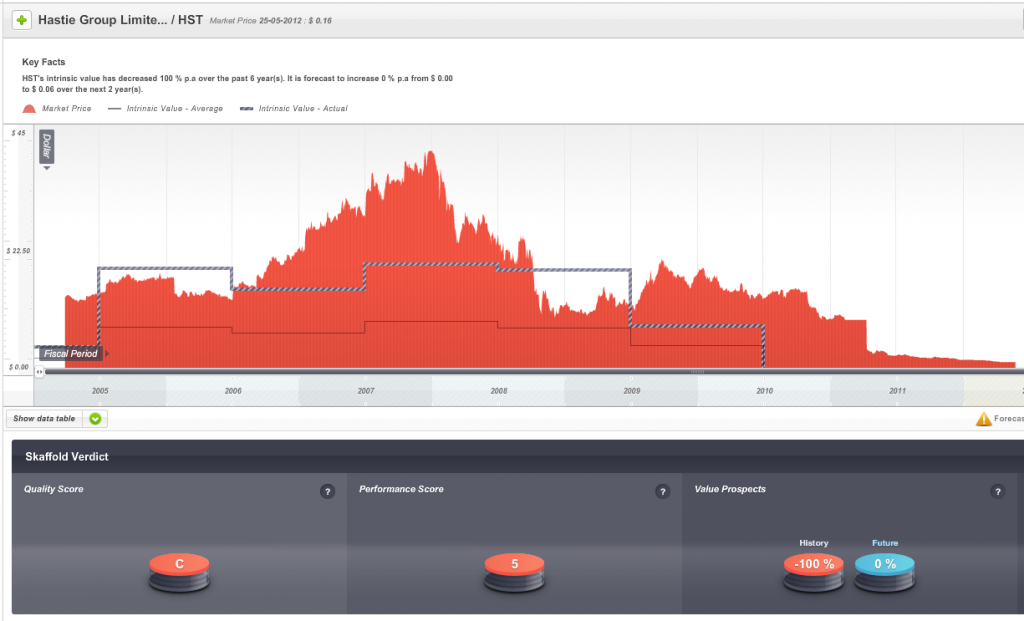
Hastie listed on the ASX in 2005 and enthusiasm for its shares once pushed the market value of the company to $500 million and to a share price – just before the GFC – of more than $40.
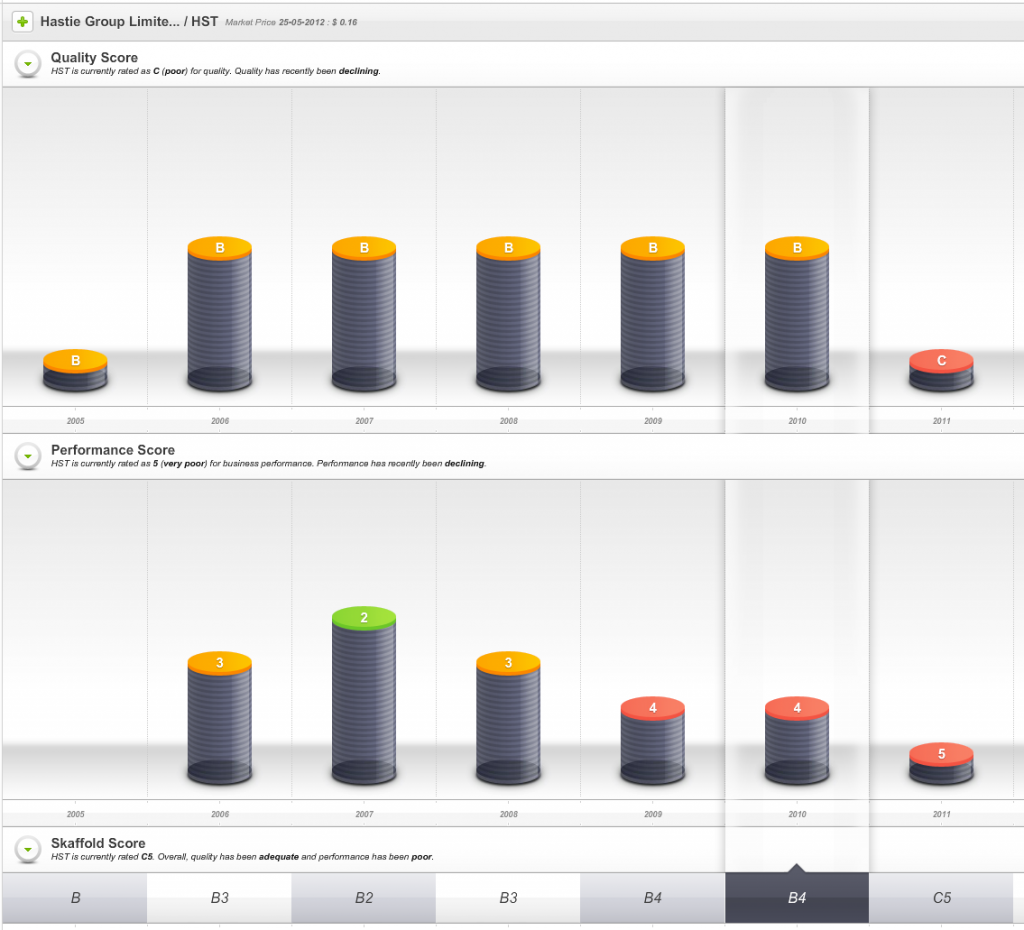
So, one sign of impending danger was that from 2009 onwards the company’s Quality Score dropped well below investment grade.
Fig 1. Skaffold Quality Score History since listing. (International Patents Pending). Hastie Group
The second warning was that the company’s shares, when they were at the height of their popularity were trading well above intrinsic value and intrinsic value was not rising at a satisfactory rate.
Fig 2. Skaffold Line. Hastie Group. (International Patents Pending). Expensive in 2006-2008 and flat and declining intrinsic value from 2007.
For many investors Figs 1 and 2 might be enough to turn the page and look to invest elsewhere. For those with more than a cursory interest however, Skaffold opens a window to the performance of the company.
As Fig 3 reveals, the company was reporting profits but even rising levels of debt failed to stem declining returns on equity.
Fig 3. Skaffold Capital History (International Patents Pending). Hastie Group. Note declining blue line (ROE) despite rising red columns (debt) and rising green line (profits)).
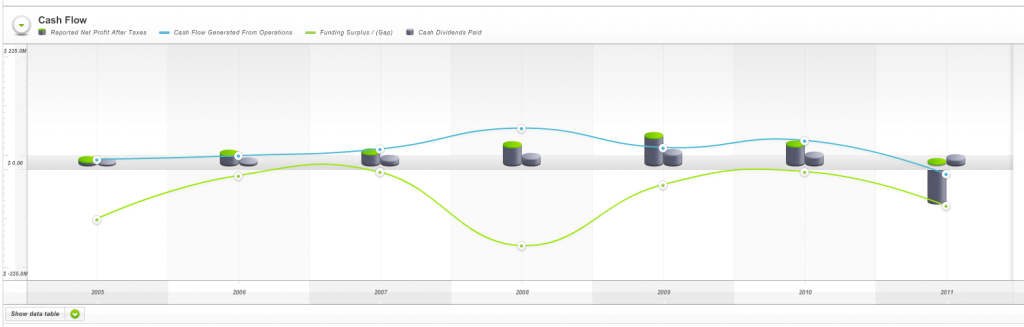
And weak cash flows (Fig 4.) as measured by Skaffold’s Funding Gap (the green line was below zero every year since listing) ensured the company would never meet all of our investment criteria.
Fig 4. Skaffold Cash Flow (International Patents Pending). Hastie Group. (note the green line (Funding Gap/Surplus) is almost always negative – biting off more than it can chew?
Finally, I note that Skaffold does something I reckon is positively spectacular. Using the Cash Flow Evaluate Screen in Fig 4. above as an example, Skaffold’s computing engine converts the chart to plain and natural English! Its a simple paragraph that explains in easy-to-understand terms precisely what you are looking at. Imagine that being updated live for every screen and for every listed stock! Have a read…
Fig 5. Skaffold’s Auto-generated plain language Cash Flow Screen description. (International Patents Pending)
Avoid potential disasters with Skaffold’s Quality Scores.
Skaffold consistently identified HST as expensive or poor quality or both.
For investors who seek to give themselves every opportunity to avoid the landmines, Skaffold’s timely assessment of HST’s poor investment quality represents the kind of early warning signal you need.
Decades of academic research into the study of corporate failure are the backbone of Skaffold. Isn’t it time you made Skaffold your portfolio’s most important investment?
To find out more about how Skaffold can help you avoid potential disasters such as Hastie Group Limited and best protect your portfolio in preparation for the future contact Donna at Skaffold on (02) 9692 5750 or register to attend the next Skaffold online webinars from the complete comfort of your own home, office or combine harvester.
Posted by Roger Montgomery, Value.able author, Skaffold Chairman and Fund Manager, 28 May 2012.
by Roger Montgomery Posted in Investing Education, Skaffold.
- 11 Comments
- save this article
- POSTED IN Investing Education, Skaffold
-
Has Wesfarmers got it right?
Roger Montgomery
May 28, 2012
 In writing Value.able I wanted to explain return on equity almost as much as I wanted to introduce the idea of future intrinsic value estimates and Walter’s intrinsic value formula.
In writing Value.able I wanted to explain return on equity almost as much as I wanted to introduce the idea of future intrinsic value estimates and Walter’s intrinsic value formula.From Value.able, PART TWO, The ABC of Return on Equity:
Return on equity is essential for value investors for so many reasons and Wesfarmers purchase of Coles was a great case study:
“In 2007, Wesfarmers had Coles in its sights. In that same year, Coles reported a profit of about $700 million. In its balance sheet from the same year, Coles reported about $3.6 billion of equity in 2006 and $3.9 billion of equity at the end of 2007. For the purposes of this assessment we will accept that the assets are fairly represented in the balance sheet. Using only these numbers we can estimate that the return on average equity of Coles was around 19.9 per cent.
Importantly, Coles has been around a long time, is stable, very mature and established and supplies daily essentials. While its prospects may not be exciting, there is the possibility that Wesfarmers may improve the performance of the Coles business.
So the target, Coles, is a business with modest debt and $3.9 billion of good-quality equity on the balance sheet that generated a 19.9% return. The simple question is: What should Wesfarmers pay for Coles? If it gets a bargain, it will add value for the shareholders of their business. If it pays too much, it will do the opposite – destroy value and perhaps its reputation.
Now, if you were to ask me what to pay for $3.9 billion of equity earning 19.9 per cent (assume I can extract some improvements), I would start by asking myself what return I wanted. If I were to demand a 19.9 per cent return on my money, I would have to limit myself to paying no more than $3.9 billion. If I was happy with half the return, I could pay twice as much. In other words, if I was happy with a 10 per cent return, which I think is reasonable, I could pay $7.8 billion, or two dollars for every dollar of equity. And finally, if I think that I could do a much better job than present management, I could pay a little more, $9.75 billion perhaps.
Now suppose you consider yourself much better at running Coles than the present Coles management. Remember, this is one of the motivators for acquisitions. Suppose you believe that you can achieve a sustainable 30 per cent return on equity. Assume you were seeking a 10 per cent return on your investment – a modest return by the way, but justified by the risks involved.
The basic formula to calculate what you should pay for a mature business, like Coles, is:
Return on Equity/Required Return x Equity
Using this formula the estimated value of Coles is:
0.3/0.1 x 3.9 = $11.7 billionEven if I thought I was a brilliant retailer, I would not want to pay more than $11.7 billion for Coles. Given the risks, I may want a higher required return than 10 per cent. If I demanded a 12 per cent required return, I would not pay more than $9.75 billion (0.3/0.12 x 3.9 = $9.75).
I will explain this formula, which represents the work of Buffett, Richard Simmons and Walter in more detail in Chapter 11 on intrinsic value.
Of course, if we think that the balance sheet is overstating the value of the assets, the result would be a lower equity component and a higher return on equity. As Buffett stated:
Two people looking at the same set of facts, moreover – and this would apply even to Charlie and me – will almost inevitably come up with at least slightly different intrinsic value figures.
The result will be modestly different but the conclusion will be the same.
With around 1.193 billion shares on issue, the above estimates suggest Coles might have been worth between $8.17 and $9.80 per share.
Now, what did Wesfarmers announce they would pay for Coles? The equivalent of about $17 per share!
What do you think would happen to your return on equity if you paid the announced $22 billion for a bank account with $3.9 billion deposited earning 19.9 per cent? Your return on equity would decline precipitously to around 3.5%.”
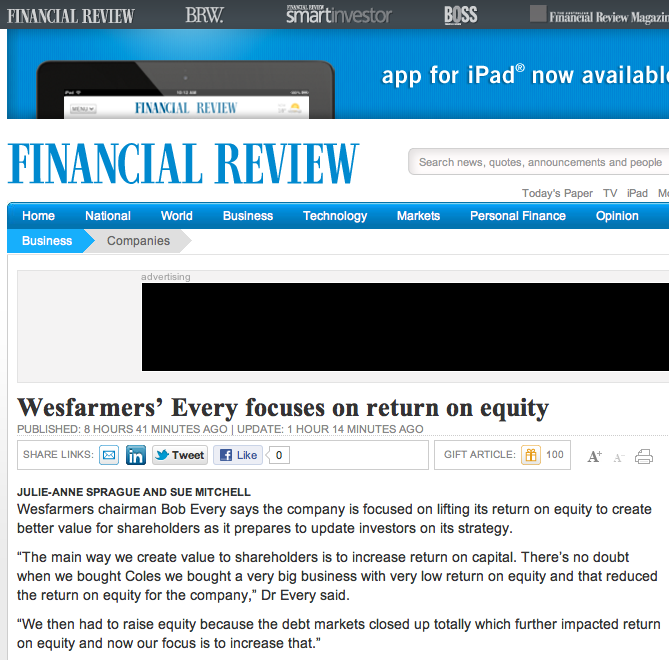
With that in mind I wonder whether the comments Wesfarmers were reported today to have made to The Financial Review (see image, I subscribe and think its great) were complete. Of particular interest is the paragraph; “The way we create value to shareholders is to increase return on capital. There’s no doubt when we bought Coles we bought a very big business with very low return on equity and that reduced the return on equity for the company.”
Assuming the comments and statistics are correct, I would argue that the reason for the decline in Wesfarmer’s Return on Equity is not because Coles had a low ROE – as Wesfarmers are reported to have suggested – but because Wesfarmers simply paid too much for Coles. Do you agree or disagree?
What are your thoughts?
Posted by Roger Montgomery, Value.able author, Skaffold Chairman and Fund Manager, 28 May 2012.
by Roger Montgomery Posted in Companies, Consumer discretionary, Skaffold, Value.able.
-

MEDIA
What Value.able Insights does Roger have on Flight Centre?
Roger Montgomery
May 9, 2012
Do Indochine Mining (IDC), Silverlake Resources (SLR), Iluka Resources (ILU), Horizon Oil (HZN), Boart Longyear (BLY), Newcrest Mining (NCM), BHP Billiton (BHP), Rio Tinto (RIO), Think Smart (TSM), New Hope Coal (NHC), Ludowici (LDW), Alumina (AMC), Flight Centre (FLT), Hawkley Oil & Gas (HAG), M2 Communications (MTU), Northern Star (NST), Codan (CDA) or Onesteel (OST) make Roger’s coveted A1 grade? Watch this edition of Sky Business’ Your Money Your Call broadcast 9 May 2012 to find out. Watch here.
by Roger Montgomery Posted in Companies, Investing Education, Skaffold, TV Appearances, Value.able.
-
Should you watch director’s dealings?
Roger Montgomery
May 8, 2012
 Once upon a time JB Hi-Fi was a category killer: its returns on equity were unassisted by debt and stratospheric and it was all reflected in a strong share price. But something has changed. I wrote previously, and commented elsewhere, that JB Hi-Fi was maturing, that returns on equity were flattening and that the sun was setting on the ability of the business to reinvest profits at the very high returns of the past. The impact of this of course is flatlining intrinsic values. Indeed take a look at the Skaffold valuation line chart below. You can see that even by 2014, JBH’s intrinsic values are expected to show no appreciation from 2009/2010. Maturity.
Once upon a time JB Hi-Fi was a category killer: its returns on equity were unassisted by debt and stratospheric and it was all reflected in a strong share price. But something has changed. I wrote previously, and commented elsewhere, that JB Hi-Fi was maturing, that returns on equity were flattening and that the sun was setting on the ability of the business to reinvest profits at the very high returns of the past. The impact of this of course is flatlining intrinsic values. Indeed take a look at the Skaffold valuation line chart below. You can see that even by 2014, JBH’s intrinsic values are expected to show no appreciation from 2009/2010. Maturity.That of course hasn’t prevented me from buying a few shares around$15.00. Fortunately however we were quick to change our mind and even secured a small profit.
I wonder whether the first signs of business performance beginning to mature, is often the point when it becomes worth watching what directors do with their shares for some further insights?
JB Hi-Fi’s CEO, Richard Uechtritz, had been at the company for a decade prior to his retirement in 2010 and those watching his share dealings may have drawn a different conclusion to those being lulled by a bullish share price.
At the outset let me say there is no impropriety in a director selling their shares and none is suggested here. Directors are free to sell shares within the bounds of their staff trading policy and are required to report their dealings to the market.
And it’s through these announcements that the investor can see what directors are doing with their shares.
On August 20, 2009, JB Hi-Fi’s CEO held 2 million shares and 627,000 options
and he exercised options to buy another 180,048 shares at $7.27. A week later, JB Hi-Fi’s CEO had sold all of shares he had just purchased the week before for an average price of $17.65.
Then, between September 2 and 3, 2009, another 500,000 shares were sold at an average price of $18.22. By now JB Hi-Fi’s CEO held 1.5 million shares (down from 2 million on AUgust 20) and 447,267 options (down from 627,000).
Skaffold’s Valuation Line Evaluate screen for JBH reveals a maturing intrinsic value – little growth and lower IV in 2014 than 2010.
To alleviate the need to read thousands of annual reports, for every listed company, going back a decade try www.skaffold.com
Now back to our regular programming…
Between August 20 and September 3, there are just 13 days – call it two weeks.
Another 174,656 options were granted on 14 October 2009, and then, in early February 2010, JB Hi-Fi announced the retirement of its CEO.. Having sold 680,048 shares in the seven months before the announcement, JB Hi-Fi’s CEO sold another 500,000 shares during the first five days of March 2010 at an average price of $19.74 leaving him with 1 million shares and 621,923 options.
In his final director’s interest notice in May 2010, the retiring CEO of JB Hi-Fi listed his direct equity interest in the company at 1 million shares and the 621,923 options. For investors who are interested in gaining a possible inside track on the prospects and potential of a business, it may be useful to watch directors’ dealings in their shares.
Of course sometimes the selling can mean nothing at all but my observation is that watching the selling offers some insights. If motivated by urgency, a desire to lock in lofty share prices or grim expectations, information about director’s selling can be more useful than watching their buying.
In April 2011 (about a year later), Richard Uechtritz returned to JB Hi-Fi as a Non-executive director. Until his return, he didn’t have director’s obligations so he was not obliged to make public any of his private share dealings. Upon his return, however, he revealed that he owned only 421,000 options. In other words, he appears to have subsequently sold the one million shares he held at the time of his retirement.
JB Hi-Fi shares do not enjoy the lofty levels they once commanded and investors who tracked the sale of shares by its CEO may have been given a prompt to look deeper into the company, its prospects or at least the impact of those prospects on its shares. Of course it could all be happenstance, company CEO’s have no particular insights and their selling is purely a reflection of the need to diversify. ANy subsequent share price declines may just be coincidental.
JB Hi-Fi’s latest results were less than spectacular and, while the company will continue to win in the race against its listed peers, the reality is its margins remain under increasing pressure, it’s losing share to the internet and its remaining store rollout plan is contributing to a maturing set of metrics. Oh, and the share price now? Just above $9.30.
So do you think you should keep an eye on director’s dealings? What have been your observations? Can you nominate some companies in which directors dealings having given you cause to pause…
Posted by Roger Montgomery, Value.able author, SkaffoldChairman and Fund Manager, 9 May 2012.
by Roger Montgomery Posted in Consumer discretionary, Skaffold, Value.able.
-
Are you being served?
Roger Montgomery
May 3, 2012
PORTFOLIO POINT: Office provider Servcorp is basking in strong earnings forecasts from analysts, but a capital raising in 2010 raises some important questions about the company.
Recently (Click here), I discussed Leighton and the quality of management’s relationship with its employees. Here I look at another company with a shareholder who holds a controlling stake, and examine its relationship with minority shareholders.
You can look at anything from a number of angles and, more often than not, reach an entirely different conclusion. Two investors, for example, presented with the same set of facts can reach polar opposite conclusions. It’s the old glass half-full versus half-empty line.
I find that having a set view and unwittingly being tied to that perspective limits one’s ability to switch fast enough when the evidence is mounting that the view might be wrong. Few are completely immune, but self-awareness is the first step to conquering any weaknesses.
The investing consequence of opening one’s mind to change is that portfolio turnover goes up. I may buy something back that I recently sold, because new evidence suggests I should, and that ‘re-purchase’ may be at a higher price. This may be seen as somewhat flaky and I agree it would be, if it were not evidence-based.
Looking at things differently, however, is necessary because it does produce fresh insights. On page 20 of Warren Buffett’s 2003 annual letter to shareholders, he wrote: “…I made a big mistake in not selling several of our larger holdings during The Great Bubble.” Shining a light – or perhaps a light with a different filter – undeniably helps our investment analysis.
With that in mind, I want to present some numbers from Servcorp’s annual reports and ask you to think about what your conclusion might be.
This service and virtual office provider has not produced growing profits since 2007.
Source: www.Skaffold.com PATENTS PENDING.
Back then, profits were $34 million, and while they grew to $39 million in 2009, they subsequently fell to $5 million in 2010 and $4 million in 2011. For the record, analysts are in aggregate forecasting profits to grow to $13 million in 2012, and $28 million and $37 million in 2013 and 2014, respectively.
So that’s one way of looking at this business and on that basis, you may be tempted to investigate the opportunity as a turnaround story. I certainly am, as any global recovery from the Euro crisis would position Servcorp well with the rollout of its floor leasing operations.
Is there another perspective?
But before I go jumping in, here’s another way to consider the information. In 2010, the full year profits plunged to $5 million, the company raised $78 million – on top of the $76 million already invested – by issuing 18 million shares. This move took shares on issue to 98 million and capital raised to $154 million. And looking at the retained earnings account for that year reveals that the balance declined by $10 million. This is something I want to pick up on.
When a company earns a profit of $5 million, as Servcorp did in 2010, retained earnings rise by this amount. Then, if dividends are paid, retained earnings goes down by the amount of the dividend. A net decline of $10 million in retained earnings after a $5 million profit suggests $15 million was paid in dividends.
One way to look at this situation is to assume that the capital raising is for growth; I will give the company the benefit of the doubt and agree. An alternative and admittedly more cynical way to look at it is to assume the capital raising might have been designed to pay for the dividend.
One response to the latter proposition and the one I am leaning towards is that the capital raising was much larger than the amount the dividend exceeded earnings by and therefore the real intention of the capital raising was, indeed, growth. In turn, one retort from a much more jaded or cynical investor could be that the capital raising was made larger to disguise the fact that a larger dividend was desired. It’s all rather circular, as you will discover in a moment.
Perhaps we might never know the thought process of the board at the time and such postulations are only speculative at best, but there are two important questions, answers to which would provide some illumination. The first would be whether the money invested does indeed lead to the growth in earnings that the analysts seem to be expecting – albeit growth that will only produce profits in 2014 that are in line with those of 2007/08. The second might be to ask whether a majority and controlling shareholder is present. Once again, we can’t prove motives, but as investors we are certainly within our rights to enquire.
In the first instance, only time will tell us whether the money is invested profitably. The cash is certainly now available to help grow the business, revenue and profits. To the credit of management, first-half 2012 profits were over $8 million. So the numbers are indeed moving in the right direction.
Regarding the latter issue of ownership, we find the managing director and CEO also owns 51% of the company. Of course, to retain control they must have participated in the capital raising during the 2010 financial year, but keep in mind it could be argued that half the dividend helped fund it.
And I always ask the following question: If a company is in need of capital, why pay a dividend? It’s a basic question and often – but not always – the answer seems to point to maintaining support for the share price, a noble (if perhaps unsustainable and diluting) goal. Directors are arguably acting in shareholder’s best interests by doing things that support the share price but it is imperative the techniques are sustainable. Ultimately the best method is a sound business.
In 2011, the company did almost the same thing as it did in 2010. While no additional equity was raised, and thus the controlling shareholder was therefore spared the requirement of writing another cheque, profits of $4 million were reported, but dividends of $16 million (51% of which went to entities associated with the CEO/MD) ensured that retained earnings fell to $59 million. The $8 million dollars (50% of the dividend) arguably further reduced the personal contribution of the majority shareholder to the capital raising.
For our fund, a return on equity now of just 7% suggests Servcorp is currently not investment grade, and its share price also appears to be expensive compared to our estimate of its intrinsic value. That would change if upgrades to guidance are provided.
While we haven’t answered the questions we posed, we have certainly raised the one we should ask management before we decide to invest. In theory the board works for the shareholders so you are within your rights to ask questions such as these of the board. For your own investing, I can only leave it to you to decide whether the cup is half-full or half-empty.
Posted by Roger Montgomery, Value.able author, SkaffoldChairman and Fund Manager, 3 May 2012.
by Roger Montgomery Posted in Insightful Insights, Skaffold.
- 25 Comments
- save this article
- POSTED IN Insightful Insights, Skaffold
-

MEDIA
Uncovering the best value stocks
Roger Montgomery
May 2, 2012
In this 2 May 2012 ASX Investor Hour presentation, Roger explains that while many investors focus solely on price, he (like Warren Buffett) selects his stocks, to buy or to sell, by comparing their current price to the value he ascribes to them. Roger discusses about his value principles and the technology he uses to filter stocks (www.skaffold.com). Watch video here and view slides here.
by Roger Montgomery Posted in Insightful Insights, Investing Education, Skaffold, TV Appearances.
-
Can JBH get its Mojo back?
Roger Montgomery
April 27, 2012
What a difference a high Australian dollar (lots of people travelling and spending their money overseas and not here), a shift to online retailing, deflation, competitors going out of business, higher petrol prices and a more cautious consumer can make in the retail space in just nine months. And few companies are more exposed to all these influences than JB Hi-Fi.
Back in August 2011, the company reported the following in their annual report;
FY11 Sales $2.96b
FY11 NPAT $134.4m
FY11 NPAT Margin 4.5%
Based on these numbers as well as company guidance for sales growth in FY12 of 8% to $3.2b, the consensus analyst view at the time was for 11% FY12 NPAT growth to $150m.
Since that time however, shareholders have suffered three profit downgrades – in mid December, mid February and another this morning.
In this morning’s trading update, management have guided analysts to an estimated NPAT of $100-$105m on sales of $3.1b. Based on this latest announcement, 2012 numbers will look like this (assuming no further downgrades);
FY12 Sales $3.10b
FY12 NPAT $102.5m
FY12 NPAT Margin 3.3%
What’s clear from these numbers is that sales revenue is growing. No immediate issues there. And despite being below the initial 8% forecast, sales are now forecast to grow by 5%. The concern however is that LFL (like-for-like) sales are negative. For the nine months, sales of mature (older established stores) are down 1.3% which means without their current expansion plans, sales targets would not be met. It’s also the main reason their initial 8% sales growth target won’t be met.
But the main issue in forecasting what the business is worth is that despite this incremental sales growth, this is not CURRENTLY being converted to the bottom line. Based on management’s forecasts, NPAT margins will be 3.3% this year vs. 4.5% in the year prior, a 26.7% margin decline in just nine months. No businesses can increase intrinsic value in such an environment.
The tide that’s currently running against JBH is very strong, no plaudits for pointing that out. But when that tide turns, could JB Hi Fi be in an even better position than it was going into the non-resource-recession (a.k.a. the seven cylinder recession of 2012). There’s certainly the possibility and the key is working out when the economy turns and whether the structural changes occurring in retail are enough to adversely impact and offset the benefits of a cyclical turnaround.
Here’s what we are watching:
· Recently management including CEO Terry Smart and Chairman Patrick Elliot have been heavy sellers of their own personal holdings in JBH. What do they know? Why are they selling?
· The retail industry is experiencing a huge shake-up. Many retailers are doing it tough and many more are exciting the space. The Good Guys was being shopped around for a private sale recently with Blackstone rumoured to be the suitor. Later denied by them. Clive Peters (now owned by JBH) and WOW Sight and Sound have gone into receivership and JB’s largest competitor Dick Smith (owned by Woolies) is set to close 100 stores by 2014. Few electronic retailser are investing in growth. The night is darkest just before the dawn so we are looking for evidence that JB Hi Fi is capturing market share in such an environment, either by making acquisitions of distressed sub-scale business or by taking over leases in locations previously unavailable to them. In QLD it appears up to $250m in sales are up for grabs as competitors close. Dick Smiths had $1.5b in sales of which an optimistic analyst would say that JBH could pick up a substantial portion of.
· Currently electronic retailers are on the back foot evidenced by store closures and liquidation sales. These participants are forced sellers of excess stock putting HUGE downward pressure on retail prices and hence profit margins. In March alone, JBH experienced a 200 bps contraction in gross margins. I was silly enough to buy two C3-PO USB keys for my kids at Christmas for $40 each but picked up another two in Brisbane a few weeks ago for $18 at a closing down Dick Smiths (my new book will be called How to Go Broke Saving MoneyTM).
Margin compression of the magnitude reported recently is unprecedented for an operator of JBH’s buying power. So we are looking for signs that the worst is over in terms of competitors closing their doors, a sure sign margins will improve or cease falling precipitously.
· We are also watching closely JBH’s move into the online space. Growth has been excellent in this segment of the business (admittedly off a low base) with an average of 965,000 website visitors each week. That’s 50.2m views per annum – 2.4 times the population of Australia. The trick of course is to convert page views to sales.
· In prior years the business has benefited immensely from positive LFL sales and also an internally funded store-rollout strategy driving new sales and sales as stores matured. This was a tailwind for the business when the number of new stores being added divided by existing stores produced a high ratio. For example when the business only had 50 stores and another 15 were opened, the proportion of stores growing and adding to sales was 30%. At present the business has in excess of 150 stores and is opening 14-15 stores per annum – a ratio of just 10% in new growth. So when you have negative LFL sales in existing and maturing stores, this is a huge drag on business momentum. We are therefore watching for signs that LFL sales stabilise or turn positive so that the business gets its mojo back.
We think it can although we are convinced the very easy money from the store roll out stage of the business along with P/E expansion has been made. Businesses with a leading market position are able to survive traumatic periods in what is a highly cyclical business and are able to absorb the effects of margin compression. Provided they can capture high levels of market share amid the tumult and cement their position as the dominant player JBH might be well positioned for the next economic recovery. One might ask whether ‘Terry and Co’ will be there when that happens.
Skaffold.com Intrinsic Value 13 year chart.
Skaffold’s conservative valuation estimate for JBH is $13.43 for 2013 as can be seen by the thin orange line in the above chart. Whether the share price now approaches that valuation or that valuation instead is revised lower and approaches the price will be determined by whether the company can harness its opportunity and when the irrational pricing associated with collapsing competitors ends. Of course after that, its success will be dependent on the depth of the impact of the structural change represented by the retail shift global and online.
Amid all of your bearishness about housing in Australia, do you think retailing conditions will pick up for JBH and its peers or not? Can you buy goods that JBH sells cheaper online?
Posted by Roger Montgomery, Value.able author, SkaffoldChairman and Fund Manager, 27 April 2012.
by Roger Montgomery Posted in Consumer discretionary, Skaffold.
-

MEDIA
Given the outlook for Chinese growth and iron-ore prices, is it time to cast a critical eye over your BHP holdings?
Roger Montgomery
April 20, 2012
Roger Montgomery discusses how and if you should respond to the impact of changing global conditions on your BHP [BHP] stock holding. Read here.
by Roger Montgomery Posted in Energy / Resources, On the Internet, Skaffold.
- save this article
- POSTED IN Energy / Resources, On the Internet, Skaffold