Sirtex test results: what they mean
Longer-term investors in Sirtex Medical Limited (ASX: SRX) might be aware that the business invests heavily – around $60m in recent years – to fund a number of large-scale studies intended to prove the efficacy of their core medical device, SIR-Spheres microspheres (SIRT). The goal is to provide the statistical evidence required to drive earlier adoption globally of their SIRT procedure in the treatment of inoperable liver cancers.
The largest of these trials from the above list is SIRFLOX: a controlled, randomised trial of SIR-Spheres in the treatment of 532 patients with metastatic colorectal cancer (mCRC). The trial will consider the impact of adding SIRT to the existing chemotherapy regimen of FOLFOX6 as a first-line therapy for patients with inoperable liver metastases.
The business, investors, the entire healthcare industry, as well as many patients, are hopeful that a positive SIRFLOX trial result will see SIRT used earlier in a the treatment process to deliver significantly better outcomes in both life expectancy and quality of life than chemotherapy alone. A positive outcome would result in a move of SIRT from a ‘salvage’ (last-line) treatment to a first- or second-line – which could benefit a materially larger number of people.
A smaller study of SIRT was conducted in Belgium in June 2010. The results were published in the Journal of Clinical Oncology, and they showed a near-doubling of the median survival rate over the current standard of care (chemo). Indeed, all the studies we have reviewed to date using SIRT at various stages of treatment have been either positive, or very positive. And as investors in Sirtex, we are backing the probability that SIRFLOX will continue in this vein.
The SIRFLOX study is currently expected to deliver its results to the market in the first quarter of 2015. At that time, a similar abstract to the one below (from the 2010 study) summarising the trial’s outcomes will become available.
For many, the results may as well be in Dutch, but even if you know nothing about biostatistics, just focusing on four elements of a trial’s results will tell you if it the results are statistically significant and enough to convince oncologists – the referring stream of medicine that deals with cancer – that their patients should be treated with SIRT earlier, and more often.
To determine if the trial (or the upcoming SIRFLOX results) are successful or a complete flop, we’ll need to analyse the data. Begin by downloading this research article from the Journal of Clinical Oncology.
Investors considering the results should, we believe, focus on these four points:
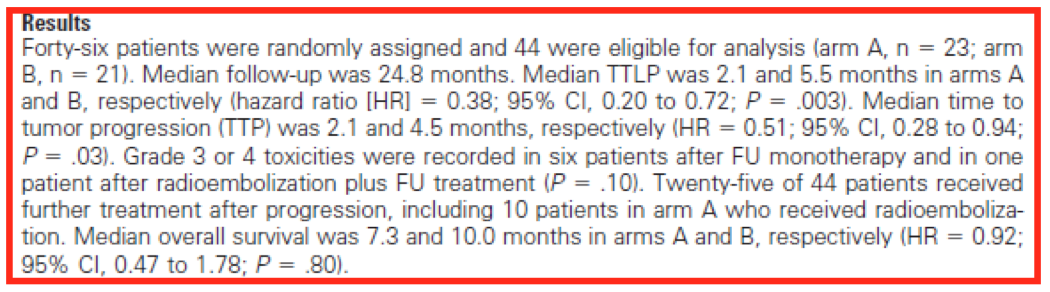
- Understand the primary end-point of the trial, which is simply what the investigators set out to examine. Concentrate on only these results, resisting the temptation to be sidetracked. In this smaller Phase III trial of SIRT combined with chemo, the primary end-point was TTLP – Time to Liver Progression in both arm A (chemo alone) and arm B (chemo plus SIRT). This is what oncologists and radiologists will be focusing on, so should you.
- Next, understand the difference between the two treatments being compared under both arm A and B. This is done by analysing the Hazard ratio (HR), which effectively defines the clinical significance of the trial. For the primary end-point, TTLP, the HR = 0.38, which means that at any point in this trial, there was a 62 per cent (1 – 0.38) lower risk of disease in the liver progressing (read: the cancer growing) under arm B (chemo + SIRT) compared to arm A (chemo alone). This is a large benefit and would likely be perceived as ‘clinically significant’, but oncologists and radiologists would require more evidence.
- Some of this would come from an understanding of the strength of the difference between the two treatments being compared under both arms A and B. A relationship measured by the trial’s P value – which simply tells us the probability that the HR of 0.38 was a complete fluke and how much confidence one can have in the trial’s results. If, for example, the P value was 0.05 (a 1-in-20 chance the result was a fluke) or higher, oncologists and radiologists would probably just throw out the results, deeming them too weak or unreliable. A strong showing here is therefore needed to back up the hazard ratio. Given the actual P value for TTLP, the primary end-point in this trial was 0.003, which means there is only a 3-in-1000 chance that the significant positive difference in the HR (0.38) was achieved by luck, such a result would be deemed to be strong and reliable.
- Finally, there is a need to understand the plausible range of the effect of the treatment under trial. This can be measured through confidence intervals. Putting in place the commonly accepted 95 per cent CI – which, for TTLP is a between 0.20 and 0.72. This means that if the Phase III trial was re-run another 100 times, the HR would fall somewhere in between 0.20 and 0.72 in 95 out of 100 trials. Another way to look at it? There’s between a 28 per cent (1 – 0.72) and 80 per cent (1 – 0.2) lower risk of liver disease progression under arm B (chemo + SIRT), compared to treatment through chemo alone (arm A). Again, very positive in terms of trial results.
And there you have it: a simple template to use when Sirtex release their SIRFLOX trial results early in 2015 to determine two things. First: if it was a success, and second: if oncologists and radiologists are more likely, in the years ahead, to add SIRT to the current standard chemotherapy regimen of FOLFOX6 as a first-line therapy in patients with inoperable liver metastases from primary colorectal carcinoma.
If similar results can be achieved by SIRFLOX’s primary end-point as reported the 2010 journal article (which was impressively positive and a reason SRX’s dosage sales continue to grow each year), we believe Gilman Wong, CEO of Sirtex, put it best:
“Sirtex is committed to delivering the Level 1 clinical evidence from large, randomized, controlled trials confirming the effectiveness of SIR-Spheres microspheres. We believe that if the results from the SIRFLOX study are positive, SIR-Spheres microspheres will be elevated to a first-line therapy for patients with colorectal liver metastases, providing important clinical benefits to patients and leading to a step-change in Sirtex’s business”. He’ll get no argument from us.
This post was contributed by a representative of Montgomery Investment Management Pty Limited (AFSL No. 354564). The principal purpose of this post is to provide factual information and not provide financial product advice. Additionally, the information provided is not intended to provide any recommendation or opinion about any financial product. Any commentary and statements of opinion however may contain general advice only that is prepared without taking into account your personal objectives, financial circumstances or needs. Because of this, before acting on any of the information provided, you should always consider its appropriateness in light of your personal objectives, financial circumstances and needs and should consider seeking independent advice from a financial advisor if necessary before making any decisions. This post specifically excludes personal advice.
INVEST WITH MONTGOMERY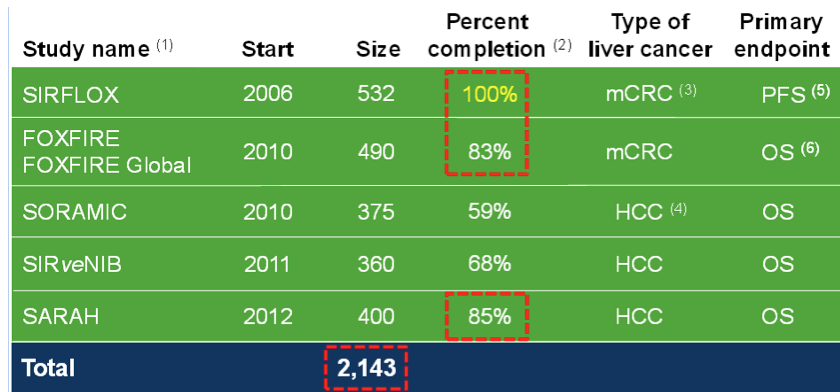

Glad it got everyone thinking. I’ll respond by stating clearly that I am only commenting on the medicine/science aspect of sirspheres, whether the company is worth investing in is outside of my expertise, I have absolutely no insights into their financials.
I stand by my previous statement, in the randomised study you describe, all patients had to have progressed or been intolerant of 5-FU, oxaliplatin and irinotecan (i.e. all the known active drugs available at that time). Thus to give patients, that had already progressed on 5-FU previously, more 5-FU is not likely to result in a response or even benefit. I would have expected no significant effect of 5-FU. And with a response rate of 0% and a PFS of 2..1 months, that is exactly what was seen. It is not that 5-FU is a placebo, it is simply that when a patient has already developed resistance to it, using it again is ineffective treatment.
The review below provides some insights into that.
Cancer Treat Rev. 2014 Jul;40(6):701-715. doi: 10.1016/j.ctrv.2014.02.006. Epub 2014 Feb 28.
A systematic review of salvage therapy to patients with metastatic colorectal cancer previously treated with fluorouracil, oxaliplatin and irinotecan +/- targeted therapy.
I take your point about recruitment times. It certainly looks as if patients have recruited more quickly to the more recent studies.
PFS is certainly an acceptable end-point in first line studies like SIRFLOX and so those results will be interesting. BUT PFS IMHO is not an acceptable end-point for an end-stage study like the one already done. There are no effective therapies that this population of patients could have received after SIRSPHERES. The issue of cross-over and effect of subsequent treatments is not likely to be relevant here. An effective treatment in this setting MUST use OS as an end-point.
Studies in similar settings:
1. Brivanib is considered a drug unlikely to ever reach daily practice – it improved PFS but not OS.
J Clin Oncol. 2013 Jul 1;31(19):2477-84. doi: 10.1200/JCO.2012.46.0543. Epub 2013 May 20.
Phase III randomized, placebo-controlled study of cetuximab plus brivanib alaninate versus cetuximab plus placebo in patients with metastatic, chemotherapy-refractory, wild-type K-RAS colorectal carcinoma: the NCIC Clinical Trials Group and AGITG CO.20 Trial.
2. Conversely Regorafenib was approved because it improved OS.
Lancet. 2013 Jan 26;381(9863):303-12. doi: 10.1016/S0140-6736(12)61900-X. Epub 2012 Nov 22.
Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial.
I do use Bevacuzimab (although yes, it doesn’t have an effect on OS), but the Bev studies were predominantly early line studies so the effect of cross-over and subsequent treatments has diluted any effect on OS (as expected). SIRFLOX and FOXIRE may have a similar problem but importantly, unlike Bevacuzimab, SIRSPHERES are not going to be appropriate for most patients in the control arm once they have progressed (unless they have only progressed in the liver – a likely minority of patients). I doubt there will be much cross-over.
It is important to understand the spectrum of colorectal cancer to understand why I think SIRT will remain a niche product. It ranges from colorectal cancer that is isolated to the colon (can be resected and cured). This is the most common type of colorectal cancer treated – this population gains no benefit from SIRT. It then progresses to patients where the cancer has metastasised to organs outside the colon, most commonly the liver and lungs. Of these, a significant number will have cancer in multiple sites in which case treating them with a liver only treatment like SIRT is not appropriate (and this is not the population that SIRFLOX or FOXIRE are testing). There is then the smaller group of patients who have liver only or liver predominant metastatic cancer. A percentage will be suitable for liver resection or ablation. This leaves a small fraction of the total population of colorectal cancer that may be suitable for SIRT. (from experience this is <<10% of all colorectal cancer patients I see)
I already use SIRT in the population of patients in which the current evidence (including the study reported) suggests there is a benefit. I, like most medical oncologists, will change practice if the evidence provided suggests that we should. But I have not seen/heard anything that suggests that the population of patients that SIRT will be suitable for is going to be large.
It is difficult to articulate all the subtleties of cancer management in a blog format and so this is simply a summary. Matthew Burge has also provided some comments above that are highly relevant and could also further complicate the issue.
You should have my phone number on file, if you would like to discuss.
P.S To Interested long-term holder, 1. a benefit of 6-12 months in PFS would be astounding and is also extremely unlikely. The study is powered to identify a much smaller difference (so even the company doesn't think we are going to see a difference that large). 2. referring patients for SIRT doesn't mean we "lose" the patient. The procedure is done by interventional radiologists (who typically don't have patients – they perform a procedure if requested by the primary clinician and once the procedure is completed, the patient returns to the medical oncologist). As SIRT is definitely not curative, it is not mutually exclusive, having SIRT doesn't prevent use of treatments usually given by medical oncologists (either before, after or during). There is no loss for me to refer patients for SIRT (financial or otherwise), it is merely a question of whether I am recommending a treatment that helps versus harms (although SIRT is generally well tolerated, it doesn't mean there are no complications or side-effects).
I am an oncologist. In cancer care we generally work in increments of benefit, not new blockbuster cures. Hence a few extra months survival from sirflox study would be highly significant and likely practice changing. There is no financial disincentive to referring patients for sirt therapy, and that would never be a consideration in any case as providing the best patient care always comes first.
Fantastic and comfort inducing Matthew.
‘If PFS = OS, but only delayed by 5-6 months, with accompanying side effects, I wonder how many oncologists would use such a treatment?’
Fair comment. As has been stated 6 months of extra life is worth the $ cost to many people all over the world.
As for side effects, yes there are some for generally a very short period. However if the alternative is more systemic chemo to deal with the liver cancer then would you consider this a better procedure? Genuine question as I believe the side effects of SIRT as a lot more tolerable than monthly chemo. Would be pleased to have a medico view on this.
I am also a medical oncologist. The FOLFOX/avastin study was never designed to evaluate overall survival- the primary endpoint was PFS- emphasising the importance of what you have stated about not getting side tracked. A second line study (Giantonio et al JCO) with OS as the primary endpoint was positive for the addition of avastin to FOLFOX. Hence avastin is a standard agent we use all the time in this disease. Interesting new data are emerging that EGFR inhibiting antibodies might be superior to avastin when combined with chemotherapy in first line-with a large head to head study to be presented next month at the ASCO annual congress in Chicago. This may mean FOLFOX/avastin is an inferior first line treatment for some patients. Whether this will dilute the impact of any positive SIRFLOX results remains to be seen. The above SIR sphere trial is very small and for sure not conclusive but does, in my opinion, provide a strong hint that these spheres are active in this disease.
The use of PFS as an endpoint is only valid if the endpoint is significantly longer (years) that the OS endpoint.
If PFS = OS, but only delayed by 5-6 months, with accompanying side effects, I wonder how many oncologists would use such a treatment?
Ask someone/anyone with a few months to live whether they’d like to try for an extra 6 months.
38 consecutive quarters of growth.
dose sales have increased from 3658 in 2009 to 7299 in 2013.
sales revenue 5 year CAGR 14.8%
dividends grown from 7c to 12c
even if this is seen by some as a “niche product”, it is still a very profitable successful company worthy of an investment.
Disclosure: long term (very) holder.
I’m a little surprised at the comment of ‘what actually matters is improvement in survival’. I’m not sure SRX have ever claimed a cure, albeit that there are examples of shrinking to the point where a surgical procedure can be performed.
My understanding, and very open to be challenged, is that it often buys some additional time. Various data sets have suggested this additional time can be a few weeks up to many months, and years in some cases.
Liver cancer from mets is generally a killer, and if one can have an extra 6/12 months to put ones affairs in order, play with the grandchildren etc, then I for one would willingly pay $50k for that extra small piece of life.
I’m sure an oncologist would also have to consider the effect of the patient – SRX treatment is often far less onerous than continuous chemo. The systemic chemo is generally a good idea to hit the potential for other areas apart from the liver, but having the liver mets under some control is a big win in its own right.
As with all medical procedures one has to ask who makes a living out of any treatment. Cardiologists often only refer to surgeons as a last resort as they ‘loose the patient’. Do oncologists make less of a living when SIRT is used? Not intended to flame anyone, but we should have visibility of various view points.
Would appreciate your considered view Roger. As investors we need to be open to all views, the good, bad and ugly. If there is a real hole in the SRX research we need to know and debate.
Its interesting hearing some of teh responses from medical professionals. Additional time is, for a patient, an genuine improvement in survival
Your analysis overlooks the importance of the end points used. The reason why the 2010 wasn’t considered at all definitive was a) tiny study of only 50 patients and b) time to liver progression is a meaningless endpoint, what actually matters is improvement in survival. In the 2010 study, they were essentially comparing SIRSPHERES to a placebo and it failed to improve overall survival. This is a concern for their studies in an earlier setting because the comparison is tougher competition. The 3rd point I would make in terms of general acceptance of their treatment is that SIRFLOX took a long time to recruit patients (opened in 2006 – not reporting until 2015), this suggests a reluctance from oncologists to recommend enrollment on the study or a very strict entry criteria that will limit the patients that the treatment will ultimately be suitable for. I think Sirtex is a good company with a nice story but they have a niche product that is likely to remain niche. Disclosure: I am a medical oncologist
Thanks Adnan. It is precisely the difference in views that makes this blog such a useful venue for investors. Thanks for sharing your insights. We’ll shortly submit a response along with some of our own questions…
Hi Adnan, it’s Russ here. I wanted to let you know that your post got everyone here talking. We thought it was well considered and appreciated you taking the time to share your counter view.
I suspect my response will frighten a few investors who may not have done the research/reading to completely understand the opportunity and the risks. To those investors reading this discussion, my apologies in advance.
To hear a well-established chemotherapy agent like 5-FU described as a placebo just doesn’t sit right with me. The point here is that this was a chemorefractory study, not a first-line study, hence the reason for SIRFLOX is not lost on me. This is a blog, not a complete summary of prior trials pros/cons. Indeed, we suspect many would take a treatment that doubled the time to progression even if survival was ultimately, and sadly, not impacted.
Regarding your comment that SIRFLOX took at long time, you are right. It did. For two reasons; 1. Bruce Gray did not fund it properly when he was in charge, and 2. there was a standard of care change to FOLFOX + Avastin, which meant that the study protocol had to be amended to include Avastin patients. This did cause a delay, and it also meant their original planned study of ~300 patients had to be expanded significantly to keep the power (finished at 532 patients). If you look at the last 2 years, the SIRFLOX trial has demonstrably accelerated and a proxy for how recently initiated trials have gone – look no further than FOXFIRE – started in 2010 and will finish this year – 490 patients in 4 years… We think that’s pretty good. The SARAH study in France will recruit in less than 3 years.
On the question of Survival – progression free survival (PFS) is a perfectly acceptable endpoint for the FDA, and most medical oncologists accept it given there are 4-5 treatment lines in mCRC which can confound overall survival (OS) – essentially a clinician can switch to anything they like once a patient has progressed in a first-line trial (as I’m sure you are aware), so it’s hard to get a clear read on survival, you need lots of pts. The irony of SIR-Spheres is that if clinicians see it is working in first-line, they might just switch all their patients to it (in other words, the “control” arm may get SIRT once they progress and are outside of the clinical trial for their chemo).
Avastin – the $4.5 billion drug in colorectal cancer showed a 5 month overall survival benefit for a no-longer-used drug combo called IFL, when there was little else around. The IRONY of that is when Avastin has been combined with modern chemotherapy FOLFOX (per the SRX trial) it DID NOT IMPROVE OS – yet it did improve PFS and the clinicians still consider it gold standard.
In other words, we believe PFS data will drive use. SRX will also provide OS data once the SIRFLOX and FOXIFRE studies have been combined and enough patients produce an effect.
Out of our interest, and for our own ongoing understanding of current treatment techniques, our enquiries suggest Avastin shows no OS benefit for FOLFOX patients. Is this your understanding? Do you administer Avastin?
I can also see that you believe that SIRT will always be relegated to the salvage setting. Even if you don’t currently think your patients would benefit, do you think it worth the risk to meet with the company, if they believe your patients would benefit? I’d be delighted to introduce you to the company’s senior management because in the end, we all want the best outcome for patients suffering such an insipid disease.